-40%
25 Pack-16 Panel Instant Urine Drug Test Cup -ETG & FENTANYL & K2 -CDOA-9165EFTK
$ 73.91
- Description
- Size Guide
Description
Product Description:Detects 16 drugs in urine
FDA 510k approved – (certifies the drug testing device passes FDA standards)
No handling or manipulation of urine needed to activate testing
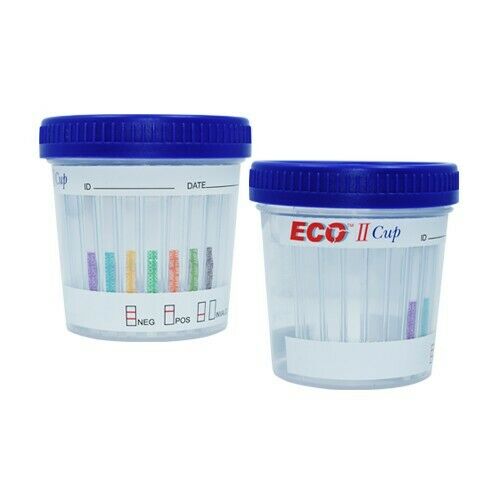
Integrated drug test cup
Perfect test kit for pre-employment, students, and medical personnel
Instructions
:
This easy to use, on-site drug of abuse test combines a collection cup and testing strips that delivers instant results, convenience, and accuracy.
AMP
,
BAR
,
BUP
,
BZO
,
COC
,
ETG
,
FTY
,
K2
,
mAMP
,
MDMA
,
MOP
,
MTD
,
OXY
,
PCP
,
THC
,
TRA
Positive:
A rose-pink band is visible in each control region and
NO
color band appears in the appropriate test region. It indicates a positive result for the corresponding drug of that specific test zone.
Negative:
A rose-pink band is visible in each control region and the appropriate test region. It indicates that the concentration of the corresponding drug of that specific test zone is zero or below the detection limit of the test.
Invalid test result:
If a color band is not visible in the control region or the color band is only visible in the test region, the test result is invalid. Another test should be run to re-evaluate the specimen.